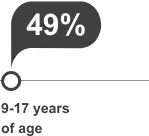
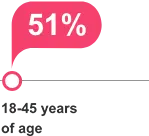
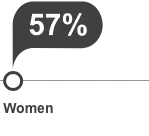
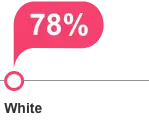
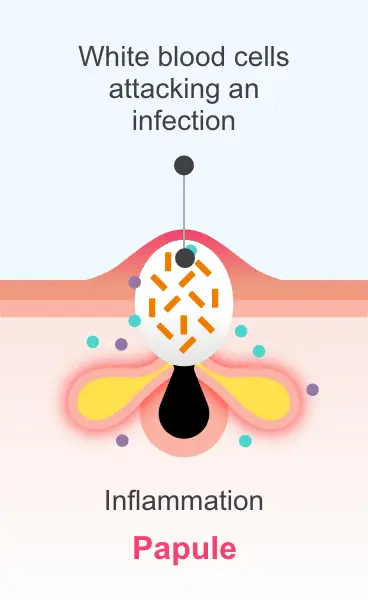
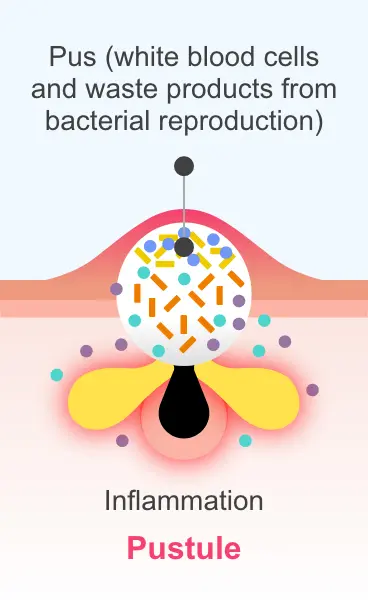
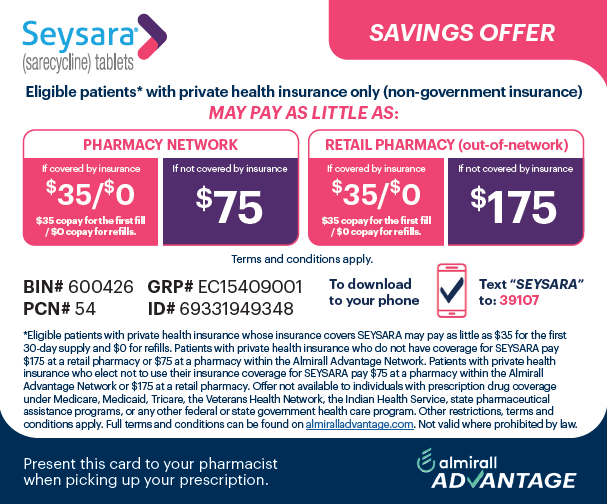
clinical significance is unknown.
P. acnes strains displayed a low propensity for the development of resistance to sarecycline.1
- At 4 to 8 times the minimum inhibitory concentration (MIC), the spontaneous mutation frequency of P. acnes in the presence of sarecycline is 10-10 (one in ten billion)1
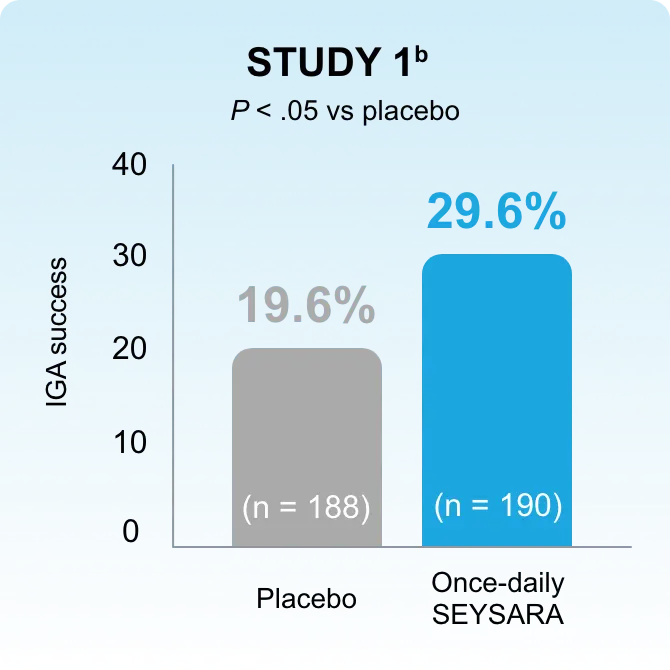
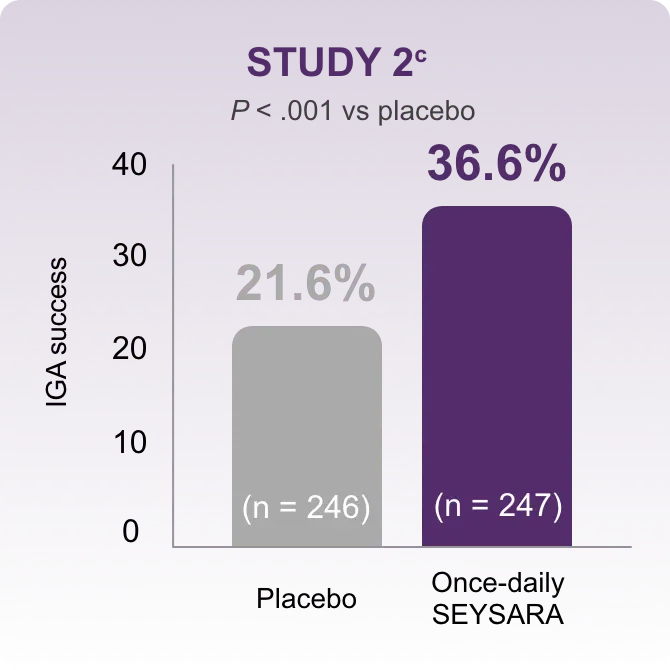
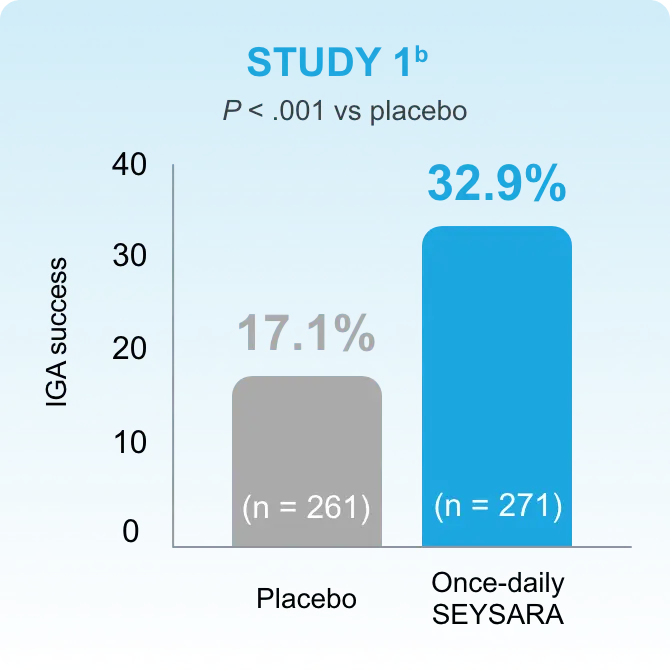
In accordance with current guidelines for antibiotic stewardship, SEYSARA provides a valid option to treat moderate to severe acne.
When prescribing systemic antibiotics, the issue of bacterial resistance remains a major concern4
Limited systemic antibiotic use is urged due to the incidence of IBD, pharyngitis, Clostridium difficile infection, and induction of Candida vulvovaginitis 4

P. acnes strains displayed a low propensity for the development of resistance to sarecycline1*
See Limitations of Use and Warnings and Precautions regarding bacterial resistance in the accompanying Important Safety Information

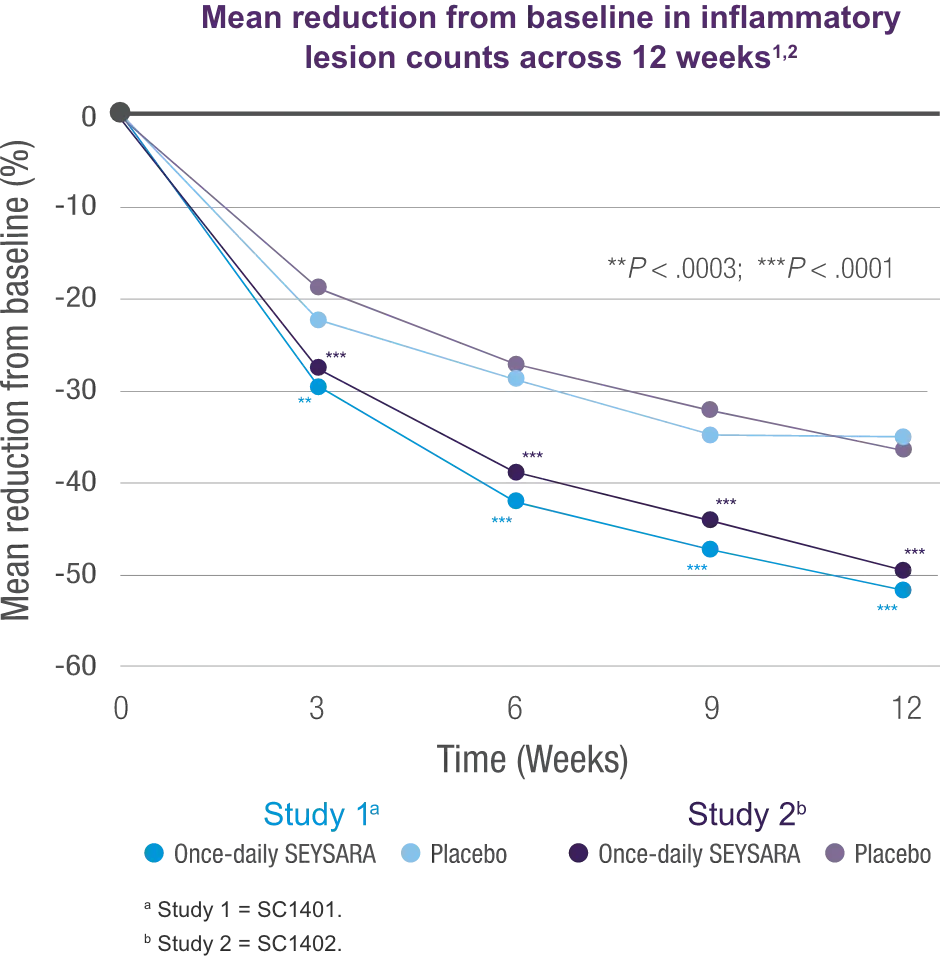
Efficacy of SEYSARA beyond 12 weeks and safety beyond 12 months have not been established
An established safety profile with low rates of GI side effects1-3
The most common adverse reaction in pivotal trials (incidence ≥ 1%) was nausea: SEYSARA (3.1%) versus placebo (2.0%).1
See Warnings and Precautions regarding C. difficile associated diarrhea (CDAD) in the accompanying Important Safety Information
Vulvovaginal mycotic infection (0.8%) and vulvovaginal candidiasis (0.6%) occurred in <1% of female SEYSARA patients1,2
See detailed safety and tolerability profile here.
AAD, American Academy of Dermatology; GI,
gastrointestinal; IBD, inflammatory bowel disease.
* With spontaneous mutation frequencies being 10−10
at 4–8 × MIC.1
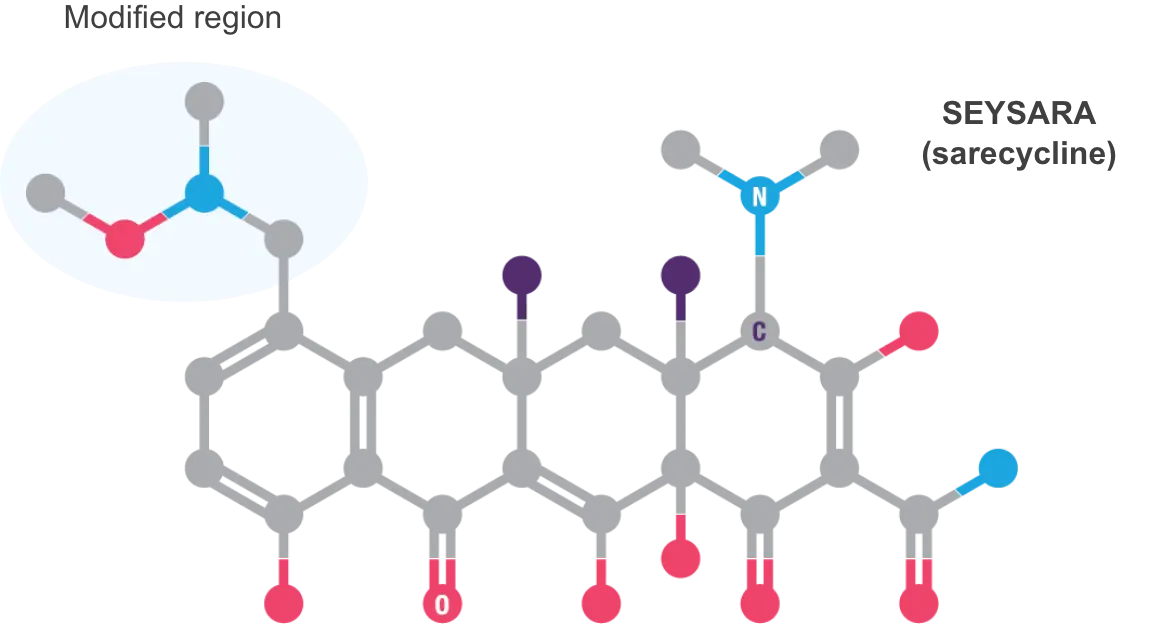
Overuse of broad-spectrum antibiotics is problematic because of their potential to induce bacterial resistance; clinical practice guidelines recommend the use of more targeted agents5
Select the correct medication, dose, and duration of treatment for your patient6

Developed specifically for the treatment of moderate to severe acne vulgaris1
P. acnes strains displayed a low propensity for the development of resistance to sarecycline1*
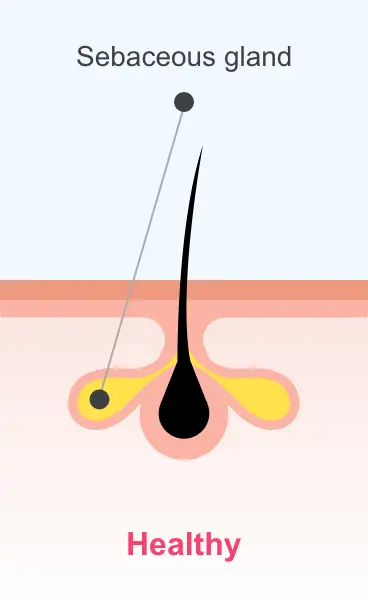
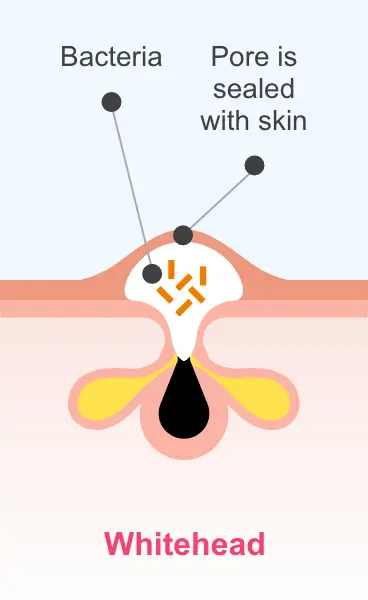
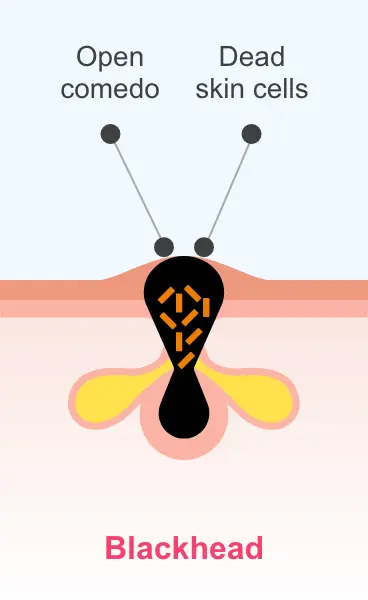
Sarecycline is active in vitro against most isolates of P. acnes; however, the clinical significance is unknown1

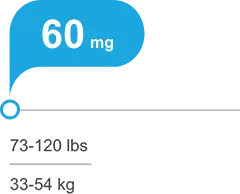
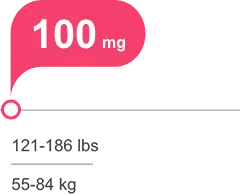
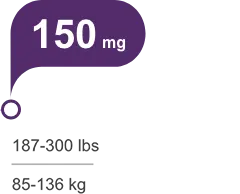
Tailored, weight-based, once-daily dosing, with or without food1

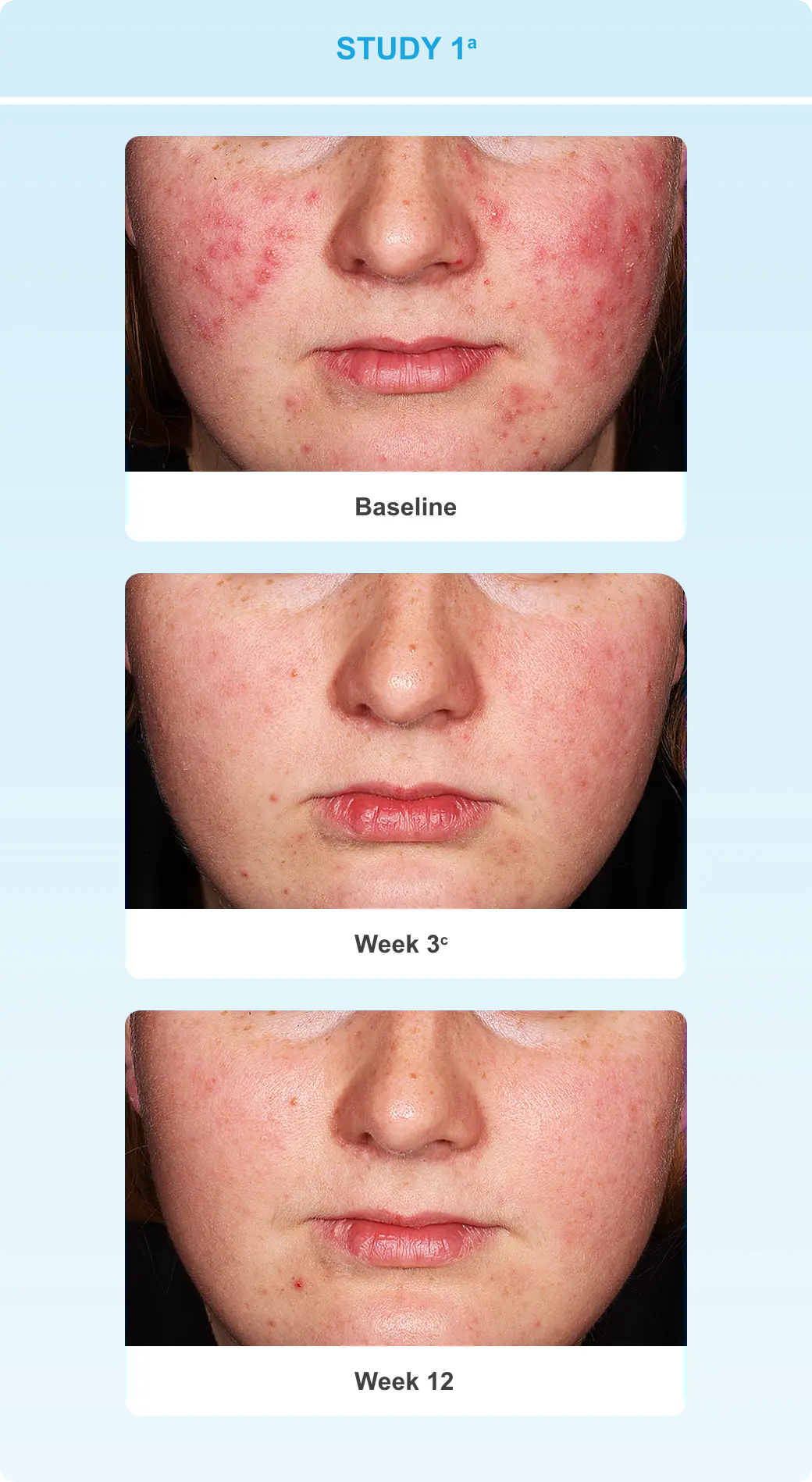
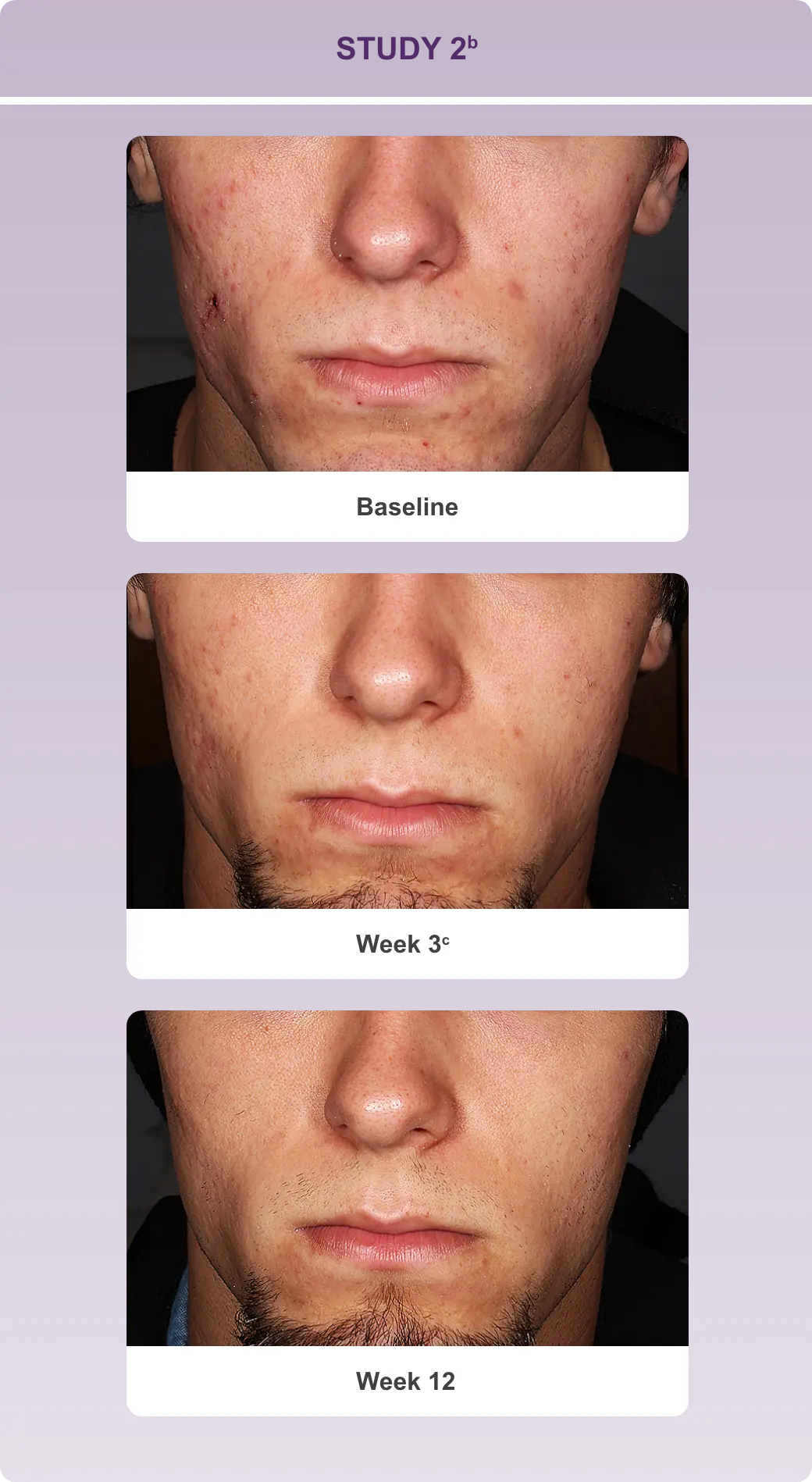
With efficacy data up to Week 12 and safety data up to 12 months1-3
CDC, Centers for Disease Control and Prevention.
* With spontaneous mutation frequencies being 10−10
at 4–8 × MIC.1

AN FDA-APPROVED LABEL DESCRIBING A LOW PROPENSITY
FOR ANTIBIOTIC RESISTANCE IN ACNE1
AAD, American Academy of Dermatology; CDC, Centers for Disease Control and Prevention; FDA, Food and Drug Administration.